CanXida recently performed a clinical trial for its frontline supplement (CanXida Remove, Formula RMV) to combat Candida overgrowth in the intestine and reduce symptoms of digestive dysfunction*. This trial was a robust scientific investigation into how CanXida Remove (Formula RMV) works, and we examined many parameters, such as Candida levels in stool and patient questionnaires about different aspects of their health. This case series highlights promising individual journeys through the 12-week trial, highlighting key areas of improvement, such as sharp reductions in Candida levels and relief from digestive and vaginal health problems. While many of our clients have had success with CanXida Remove (Formula RMV) after taking it for 12 weeks, others have only seen effects after a longer course of supplementation. Thus, it’s essential to keep in mind that 12 weeks may not be enough for everyone, especially if you have been suffering from chronic Candida issues for decades. It’s also important to emphasize that this was a controlled scientific study and was not based on surveys, anecdotal evidence, or client reviews.
Methods
This anonymized study recruited women between 18 and 50 years of age with self-reported Candida overgrowth symptoms. The participants took CanXida Remove (Formula RMV) or a placebo (fake treatment) daily for twelve weeks. They completed questionnaires assessing general and specific health parameters at three time points: the start of the trial, after six weeks, and at the trial’s conclusion (twelve weeks). These assessments covered three broad areas:
-
- Vaginal health
- Digestive health
- Mental health
Participants also provided stool samples before and after the trial and reported the quality of their bowel movements on a seven-point scale (bristol stool chart). Before the study began, participants were also asked why the study was important to them.
The amount of Candida in participant stool samples was determined using quantitative polymerase chain reaction (qPCR), a routine clinical, biological, and forensic research technique. This method allows for the specific detection of tiny amounts of Candida genetic material. For this study, we examined levels of the most medically pervasive Candida species, Candida albicans. The qPCR results are reported as colony-forming units (CFU) per milliliter (CFU/mL). CFU simply means the number of Candida albicans cells that are capable of reproducing and forming a separate colony. It is acceptable to interpret CFU/mL as the number of Candida albicans present, i.e., a higher CFU means more Candida, and a lower CFU indicates less Candida.
This trial used triple-blinding, meaning participants, researchers, and the CanXida team analyzing the results did not know who received the real treatment (CanXida Remove, Formula RMV) or the placebo (fake treatment) until the results were fully analyzed. This approach helped prevent bias and ensured more reliable results.
We selected participants for this case study based on their positive response to CanXida Remove (Formula RMV). We prioritized cases based on the reduction in the levels of Candida in their stool samples, which gave us the clearest indication that the product worked as intended. Furthermore, this allowed us to associate reduced levels of Candida with improvements in other areas. It’s important to note that these results do not prove that lower Candida levels caused the other symptoms to improve. However, based on our experience and the work of other researchers, there is likely a causal relationship between Candida levels and other health parameters in some instances.
CanXida Remove (Formula RMV) Trial Cases
Participant A:
Sex: Female
Country: USA
Before the Trial: Before the trial began, participant A described her overall vaginal health as “fair” and her overall gastrointestinal health as “poor”. Her mental health parameters were also on the lower end of the scale, with her ability to focus “very poor” and energy levels “very low or none at all”.
Why is this study important to you? This study is important to me as I struggle with yeast inside and outside of my body, the worst being in my mouth.
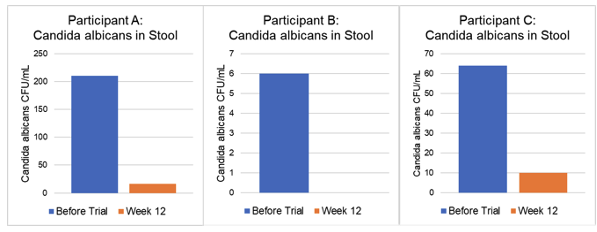
Candida albicans in stool: 210 CFU/mL
Stool Consistency: Type 6 – Mushy consistency with ragged edges
Halfway (6 weeks): After six weeks of taking CanXida Remove (Formula RMV), participant A was already seeing gradual improvements in all areas of her health. She still considered her vaginal health as “fair” but her gastrointestinal health improved from “poor” to “fair”, her ability to focus improved from “very poor” to “poor” and her energy levels improved from “very low or none at all” to “low”. These results indicated a small but noticeable improvement across several general health parameters.
Stool Consistency: Type 6 – Mushy consistency with ragged edges
After the Trial (12 weeks): After twelve weeks of taking CanXida Remove (Formula RMV), participant A continued to see improvements across broad aspects of her health. She considered her vaginal health as “good” for the first time since beginning the trial. Her gastrointestinal health was “fair,” which was an improvement from the baseline. Her ability to focus improved from “very poor” to “fair” after twelve weeks, and she described her energy levels as “moderate” which was a big improvement from “very low or none at all” when the trial began.
Candida albicans in stool: 16 CFU/mL – 92% Reduction in Candida albicans levels
Stool Consistency: Type 6 – Mushy consistency with ragged edges
Participant B:
Sex: Female
Country: USA
Before the Trial: Before beginning the trial, participant B described her overall vaginal health and gastrointestinal health as “fair”. Her mental health parameters were also on the lower end of the scale, and she described herself as feeling tired 2 to 5 times per week.
Why is this study important to you?: I’d like to improve both my vaginal and gut health and experience itchiness sometimes
Candida albicans in stool: 6 CFU/mL
Stool Consistency: Type 1 – Separate hard lumps
Halfway (6 weeks): After six weeks of taking CanXida Remove (Formula RMV) participant B saw broad improvements across different health parameters. She described her vaginal and gastrointestinal health as “good” compared to “fair” at the baseline. Several mental health parameters improved, and she described herself as feeling tired once per week as opposed to 2 to 5 times per week before beginning the trial.
Stool Consistency: Type 4 – like a smooth, soft sausage or snake
After the Trial (12 weeks): After twelve weeks of taking CanXida Remove (Formula RMV) participant B maintained her improvements in vaginal and gastrointestinal health, but some of the mental health parameters returned to their baseline levels. She strongly agreed that the product supported her vaginal health and expressed a desire to continue using the product after the trial.
Candida albicans in stool: 0 CFU/mL – Candida albicans undetectable after 12 weeks
Stool Consistency: Type 1 – Separate hard lumps
Participant C:
Sex: Female
Country: USA
Before the Trial: Before the trial began, participant C considered her vaginal and gastrointestinal health to be “good,”. However, she rated her mental well-being and ability to focus as “poor”.
Why is this study important to you? Hopefully get the relief I’ve been needing.
Candida albicans in stool: 64 CFU/mL
Stool Consistency: Type 1 – Separate hard lumps
Halfway (6 weeks): After six weeks of taking CanXida Remove (Formula RMV), participant C saw improvements in her vaginal health, which went from “good” to “excellent”. Her gastrointestinal health remained “good”, her mental well-being improved from “poor” to “good”, and her ability to focus improved from “poor” to “fair.”
Stool Consistency: Type 4 – like a smooth, soft sausage or snake
After the Trial (12 weeks): At the end of the twelve weeks, participant C maintained “excellent” vaginal health and “good” overall gastrointestinal health. She also maintained her improvements in her ability to focus from week 6, and her overall mental well-being was “fair”, an improvement from the baseline (before the trial).
Candida albicans in stool: 10 CFU/mL – 84% Reduction in Candida albicans levels
Stool Consistency: Type 4 – like a smooth, soft sausage or snake
Results
This section summarizes the important findings across all three participants. The “CanXida Effect” column indicates how CanXida Remove (Formula RMV) influenced these parameters throughout the trial.
Stool Analysis
Candida albicans Levels in Stool:
| Candida albicans CFU/mL | ||||
| Before Trial | Week 12 | Candida Reduction (%) | CanXida Effect | |
| Participant A | 210 | 16 | 92 | Improvement |
| Participant B | 6 | 0 | 100 | Improvement |
| Participant C | 64 | 10 | 84 | Improvement |
Stool Quality:
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Type 6 – Mushy consistency with ragged edges | Type 6 – Mushy consistency with ragged edges | Type 6 – Mushy consistency with ragged edges | No Change |
| Participant B | Type 1 – Separate hard lumps | Type 4 – like a smooth, soft sausage or snake | Type 1 – Separate hard lumps | Improvement at 6 weeks |
| Participant C | Type 1 – Separate hard lumps | Type 4 – like a smooth, soft sausage or snake | Type 4 – like a smooth, soft sausage or snake | Improvement |
Vaginal Health
Overall Vaginal health:
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Fair | Fair | Good | Improvement |
| Participant B | Fair | Good | Good | Improvement |
| Participant C | Good | Excellent | Excellent | Improvement |
How severe is any itchiness in the vulva or vaginal area that you experience?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Moderate | Moderate | Moderate | No Change |
| Participant B | Moderate | Not Noticeable | Barely Noticeable | Improvement |
| Participant C | Distracting | Barely Noticeable | Barely Noticeable | Improvement |
How often do you experience extra vaginal dryness OR extra vaginal discharge?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Often | Often | Often | No Change |
| Participant B | Often | Never | Rarely | Improvement |
| Participant C | Often | Sometimes | Never | Improvement |
How embarrassed are you about your vulvar or vaginal symptoms?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Very Much | Very Much | Somewhat | Improvement |
| Participant B | Somewhat | Not At All | Not At All | Improvement |
| Participant C | Extremely | Very Much | Very Much | Improvement |
Gastrointestinal Health
Overall gastrointestinal health
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Poor | Fair | Fair | Improvement |
| Participant B | Fair | Good | Good | Improvement |
| Participant C | Good | Good | Good | Maintained Positive |
Have you been bothered by HARD STOOLS during the past week?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Mild discomfort | Minor discomfort | Minor discomfort | Improvement |
| Participant B | Minor discomfort | No discomfort at all | Minor discomfort | Improvement |
| Participant C | Severe discomfort | No discomfort at all | Moderate discomfort | Improvement |
Have you had the sensation of not completely emptying the bowels when going to the toilet during the past week?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Moderately severe discomfort | Moderate discomfort | Moderately severe discomfort | Improvement |
| Participant B | Mild discomfort | No discomfort at all | Mild discomfort | Improvement |
| Participant C | Moderately severe discomfort | No discomfort at all | Minor discomfort | Improvement |
Mental Health
In the past week, how would you rate your overall energy levels?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Very low or none at all | Low | Moderate | Improvement |
| Participant B | Low | Moderate | Low | Improvement at 6 weeks |
| Participant C | Low | Moderate | Low | Improvement at 6 weeks |
In the past week, how would you rate your overall mood?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Poor | Poor | Fair | Improvement |
| Participant B | Fair | Good | Fair | Improvement at 6 weeks |
| Participant C | Poor | Fair | Poor | Improvement at 6 weeks |
In the past week, how would you rate your mental well-being?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Poor | Poor | Fair | Improvement |
| Participant B | Fair | Good | Fair | Improvement at 6 weeks |
| Participant C | Poor | Good | Fair | Improvement |
In the past week, how would you rate your ability to focus?
| Before Trial | Week 6 | Week 12 | CanXida Effect | |
| Participant A | Very Poor | Poor | Fair | Improvement |
| Participant B | Fair | Good | Fair | Improvement at 6 weeks |
| Participant C | Poor | Fair | Fair | Improvement |
Opinions on CanXida Remove (Formula RMV)
| Week 6 | Week 12 | |||||
| Participant | A | B | C | A | B | C |
| Vaginal Health | ||||||
| This product has helped to optimize my vaginal health. | Neither agree or disagree | Agree | Strongly agree | Neither agree or disagree | Agree | Neither agree or disagree |
| This product has supported my vaginal health. | Neither agree or disagree | Strongly agree | Strongly agree | Neither agree or disagree | Agree | Neither agree or disagree |
| My vaginal odor is less severe since using this product. | Neither agree or disagree | Strongly agree | Strongly agree | Neither agree or disagree | Agree | Neither agree or disagree |
| I think that this product supports a healthy vaginal microbiome | Neither agree or disagree | Strongly agree | Strongly agree | Neither agree or disagree | Agree | Neither agree or disagree |
| I have less swelling and redness in my vulvar and vaginal area since using this product | Neither agree or disagree | Strongly agree | Strongly agree | Neither agree or disagree | Agree | Neither agree or disagree |
| General Feedback | ||||||
| I would recommend this product to my friends and family | – | – | – | Neither agree or disagree | Neither agree or disagree | Neither agree or disagree |
| I would like to continue using this product | – | – | – | Neither agree or disagree | Agree | Neither agree or disagree |
Discussion
Taken together, these results indicate that CanXida Remove (Formula RMV) can help to improve a wide range of symptoms across vaginal, digestive, and mental health. The effect on Candida albicans levels in stool is a particularly striking result and vindicates the current literature that supports the anti-Candida effect of CanXida Remove’s (Formula RMV) ingredients. The results also clearly demonstrate the variety of benefits that CanXida Remove (Formula RMV) can have depending on individual circumstances.
It is important to look at how each individual benefited relative to their starting point rather than comparing their progress to that of other participants. For example, going from “poor” to “fair” on a given parameter could be a massive milestone for individuals with chronic health issues. Furthermore, just because these participants saw results at 12 weeks doesn’t mean everyone else will. Everybody has an individual battle to fight; some take longer than others. Another point worth keeping in mind is that vaginal, digestive, and mental health are multifactorial, meaning they are affected by many factors besides Candida levels. Thus, we should not consider CanXida Remove (Formula RMV), or even the lowering of Candida levels in the gut, as a magic bullet that will resolve any health issue. Instead, we should consider this supplement as one to help manage Candida-related problems as part of a larger plan. Factors such as diet, lifestyle, and genetics undoubtedly play a role in various aspects of our health. Addressing these variables goes far beyond the scope of this trial, which is the first step towards deepening our understanding of how CanXida Remove (Formula RMV) improves people’s lives.
We have highlighted three promising cases in this report, but these individuals belong to a larger cohort of participants who experienced varying degrees of success with CanXida Remove (Formula RMV) during the trial. This case study, at a minimum, provides scientifically controlled evidence that CanXida Remove (Formula RMV) can reduce the levels of Candida in the gut of certain individuals and this effect coincides with improvements across different domains of health. Further testing is underway to deepen our understanding of CanXida’s health-promoting effects.
If you are interested in learning more about Candida, check out our YouTube library, which has thousands of videos covering various Candida-related topics. You can learn more about our products by visiting our website.
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.